25.05.2021
Attitudes towards the purchase of electric vehicles ... mehr
Attitudes towards the purchase of electric vehicles in Germany over time
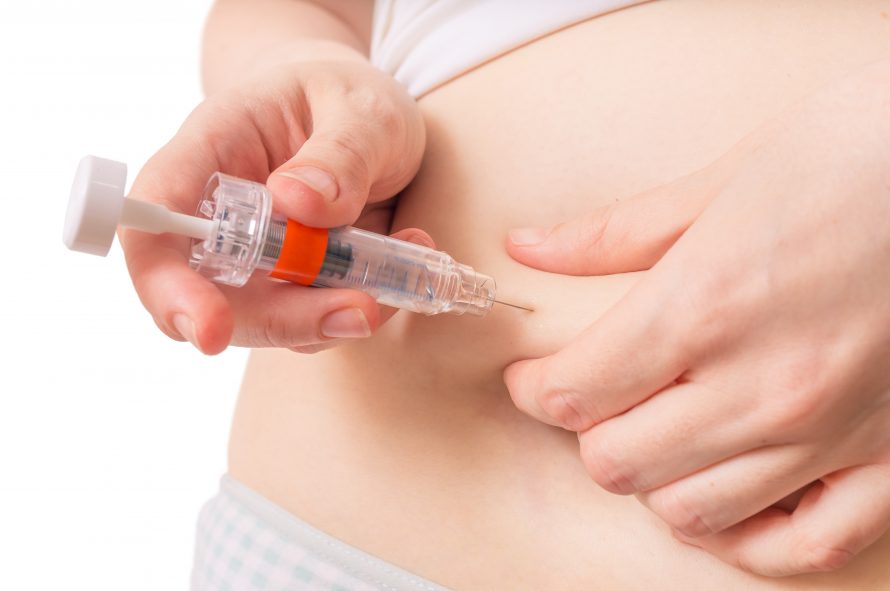
Self-injection devices used in chronic care today cover a wide range of indications from rheumatoid arthritis, osteoarthritis, Crohn’s disease, diabetes to bleeding disorders to name a few. And in the end it doesn’t matter whether the device was developed for acute or chronic care, the key features which must be established are the same for both – providing optimal treatment and /or alleviating pain with a safe, reliable and intuitive device.
So what’s the difference? With functionality and rapid improvement being at the core of acute or emergency treatment, patients self-injecting for chronic conditions are not driven by urgency and as such non-functional criteria are often key triggers in decision-making. Medical device testing research for chronic conditions is interestingly showing preferences for more technological or aesthetically appealing devices even though use is often more complex and the error ratio can be considerably higher, even after several trial runs.
And why does this matter? With the focus on patient-centric care today, enhancing the patient experience promotes patient adherence, improves treatment outcome, motivation and ultimately increases product loyalty. Despite other devices being easier to handle, the “good-looking” or “connected” devices are often first choice. And why should they not be? The “aesthetic” and/or “wireless components as part of the selection process when buying a kitchen gadget, a car or furniture are also increasingly becoming key motivational triggers in medical device selection. The user often takes functionality for granted and is even willing to compromise on convenience for a more appealing device.
And it’s not only having a device which is highly clinical in appearance, but also storage including refrigeration to which others in the household or visiting would have access to causes distress for some,. Patients do not want to be reminded or questioned about their condition or treatment in untimely settings.
Designing a device which resonates with a specific target audience and at the same time can be managed efficiently under varying circumstances requires looking outside of a controlled test environment.
To truly understand the ease of handling devices and the triggers for patient preferences, our experiences have shown that empirical testing outside of a controlled setting can provide highly targeted and valuable insights on device design based on real world evidence.
Ethnographic studies in the respondent’s home or our immersive PsyDive engagement through custom-online communities in which the administration is filmed and uploaded securely by patients (or their caregivers) provides a more holistic evaluation of the individual steps involved from preparation to cleaning up, injection and waste management. It also allows MedTech companies to zero in on “real life” concerns which do not always present themselves in in-facility interviews. Safeguarding prior to launch that self-injection devices also deliver in a real world setting can avoid costly design changes later on.
Being outside of a controlled environment requires multi-tasking resulting from any number of disruptions in the home (other family members, phone calls, pets, door bell, etc.) which can in many instances lead to mental confusion and/or lack of concentration. In such situations, clumsiness often sets in; patients become fidgety and often frustrated. As a result the prototype scoring best in a controlled environment is not capable of upholding its pole position in a “real life” setting.
You would think it can’t be so difficult with patients having IFUs to fall back on when unable to remember next steps. User instructions, however, do not always meet the mark in providing the intended support. Some are overly complex, others non-intuitive, while others are just simply poorly written or the font size is illegible to the human eye. And it doesn’t end here – what also needs to be factored in is human fallibility. The aversion to instruction manuals resulting from past experience or believing that common sense will get you through is a clear sign that more attention and testing is required. Smart technologies such as QR codes with links to tutorials or videos need to further enrich the patient experience so that the intrinsic motivation which comes with the preferred device translates into error-free administration and better treatment outcomes.
To find out more, please contact:

22.02.2024
Psyma+Exevia Health wird in diesem Jahr verstärkt smarte Lösungen für den OTC Markt anbieten. Diese Lösungen zeichnen sich durch ein klares, schnörkelloses Design aus mit ... mehr

09.10.2023
Konzept- und Kommunikationstests, Patient Journey Research, Packungstests - um nur ein paar Beispiele zu nennen - schnell durchführen und Einsichten in Echtzeit generieren. Das ist ... mehr

03.08.2020
Benutzerfreundliche Medical Devices mit höchstmöglicher Praxistauglichkeit bringen klare Wettbewerbsvorteile und sichern eine starke Positionierung im Markt. Basis für den Erfolg ist die user-zentrierte Entwicklung. Anwender ... mehr